PRODUCTS BEING SOLD ARE FOR LABORATORY / EDUCATIONAL USE ONLY. THEY ARE NOT TOYS. THEY ARE NOT FOR USE BY CHILDREN 12 & UNDER.
If you are a Company, School or University we also accept Purchase Orders. Please call us at 936-727-5038. We are owned by LAB.SUPPLY Inc and any invoices or credit card charges will reflect the same.
Chemicals that we sell are NOT FOOD / USP grade. If you buy a Chemical and you are using it for Clinical / Human (Animal) Use / Application we are not liable. Again they are not for or on Human / Animal use / testing.
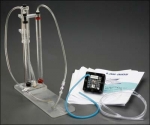
Investigating Hydrogen Fuel Cells

Categories
- Agricultural Science Pathway Kits
- Astronomy (4)
- Atomic, Molecular Models (22)
- Balances, Scales & Weights (1)
-
Biology
- Biology Kits (68)
- Biology Lab Supplies (18)
- Displays & Exhibits (41)
- Dissection Material and Supplies
- DNA (9)
- Incubators
- Microbiology
- Microscope Slides, Plain and Prepared (32)
- Models Biology (1)
- Owl Pellets (6)
- Physiology (2)
- Skeletons (16)
- Slide Making Equipment (1)
- Soil Testing (21)
- Specimens
- Tomatoes
- X-Rays (4)
- Charts and Posters
- Chemicals
- Einstein Data Logger & Interface
- Earth Science (87)
- Electrophoresis (21)
- Energy (18)
- Entomology
- Environmental Science (22)
- Geology
-
Glassware
- Beakers
- Bell Jars (7)
- Bottles and Jars (72)
- Burettes / Burets (29)
- Centrifuge Tubes (7)
- Condensers (31)
- Crystallizing Dishes (6)
- Dessicator Glass (1)
- Distillation / Distilling Appts.
- Droppers Glass (19)
- Drying Tubes (6)
- Flasks
- Funnels Glass (6)
- Glassware Sets and Kits (42)
- Graduated Cylinders
- Misc. Glassware (34)
- Petri Dishes (7)
- Pipettes / Pipets (25)
- Retorts Glass (3)
- Separatory Funnel (17)
- Stirring Rods (4)
- Test Tubes (15)
- Tubing Glass (11)
- Vials Glass (7)
- Watch Glasses (2)
- Green Science (39)
-
Hanna Instruments
- Backpack Chemical Test Kits
- Dissolved Oxygen Meters
- Electrical Conductivity Meters
- Electrodes, Probes, & Sensors
- Handheld Colorimeters
- Ion Selective Electrode Meters
- Light Meters
- Magnetic Stirrers
- Oxidation Reduction Potential Meters
- pH Meters (1)
- Refractometers
- Salinity Meters
- Solutions
- Temperature Testers
- Titrators
- Total Dissolved Solids Meters
- Turbidity-Meters
-
Innovating Science Kits
- AP Biology (10)
- AP Chemistry (31)
- Biotechnology (9)
- Chemical Inventory Management (1)
- Chemical Sets (13)
- Chemistry & Measurement (7)
- Chemistry Demonstration Kits (42)
- Consumer Chemistry (7)
- Forensic Chemistry (20)
- General Chemistry (13)
- Green Chemistry (18)
- Life Science (32)
- Microchemistry (47)
- Safety Clean-Up Kits (4)
- Kemtec Kits (1)
-
Kits All Types
- AP Biology (11)
- AP Chemistry Kits (50)
- Area 51 (1)
- Bacteria (5)
- Biodiesel (3)
- Biology General (104)
- Biotechnology (17)
- Blood Analysis (32)
- Chemical Sets (15)
- Chemistry And Measurement (65)
- Chemistry Demonstration Kits (96)
- Chemistry General (111)
- Consumer Chemistry Kits (15)
- Earth Science (23)
- Environmental Chemistry Kits (31)
- Food Analysis (11)
- Forensic Chemistry Kits (52)
- General Science (21)
- Green Chemistry Kits (22)
- Marine Biology (7)
- Microchemistry (51)
- Microchemistry Lab Hardware (14)
- Outbreak (1)
- Physics (52)
- Polymer Chemistry Kits (2)
- Safety Clean-Up Kits (4)
- Urine (4)
- Water Analysis (21)
- Lab-Aids Kits
-
Lab Equipment & Supplies
- Aprons (7)
- Autoclaves (5)
- Balances Scales And Weights
- Baths Dry
- Brushes (6)
- Buret Burette Clamps (14)
- Burner Accessories (18)
- Burners (19)
- Centrifuges (23)
- Clamp Holders (14)
- Clamps (41)
- CO2 Carbon Dioxide Meters (3)
- Colony Counter (5)
- Cork Borers (5)
- Cork Boring Machine (4)
- Dissolved Oxygen Meters (2)
- Drying Racks (3)
- Electrical Conductivity Meter (8)
- Electrodes, Probes, & Sensors (9)
- Filter Paper
- Filtering Kits (3)
- Fume Hoods & Accessories (32)
- Gloves
- Goggles Safety (12)
- Heating Mantles (53)
- Hot Plates / Stirrers (55)
- Incubators
- Inoculating Loops (6)
- Ion Selective Electrode Meters (1)
- Kits and Sets (22)
- Lab Carts (2)
- Lab Coats (4)
- Lab Jacks
- Light Meter (1)
- Magnifiers (28)
- Melting Point Apparatus (1)
- Micro-Pipettes Digital (19)
- Miscellaneous Lab Equipment (25)
- Mixers and Shakers
- Ovens
- Oxidation Reduction Potential Meters (5)
- Petri Dishes (8)
- pH Meters
- Pi-Pumps (13)
- Pipette Fillers (21)
- Pipettors (18)
- Pumps
- Refractometers
- Refrigerators / Freezers (1)
- Rubber Stoppers
- Salinity Meters (1)
- Sealing Film (1)
- Solutions
- Spatulas Scoops (38)
- Spectrophotometers
- Stir Bars / Spin Bars (52)
- Stirrers / Hot Plates (55)
- Stirring Rods, Stir Rod (5)
- Support Rings (17)
- Support Stands (20)
- Support Stands Ring Stands and Acc. (31)
- Temperature Tester (12)
- Test Papers (6)
- Test Tube Racks / Stands (16)
- Thermometers (34)
- Titrators (2)
- Tongs (5)
- Total Dissolved Solids Meters (2)
- Transfer Pipettes Pipets (9)
- Tubing
- Turbidity Meters
- Ultrasonic Cleaners (3)
- Vials (7)
- Wash Bottles (10)
- Watch Glasses (2)
- Water Baths (9)
- Water Purification (3)
- Water Purification Distillation (11)
- Wire Gauze (6)
- Wires (30)
- Meteorology (9)
- Microchemistry (29)
- Microscopes
- Neo/Sci Kits
- NewPath Learning
-
Physics
- AP Physics (2)
- Cathode Ray DischargeTubes
- Density, Specific Gravity (51)
- Electricity
- Electrolysis (5)
- Electromagnetism (16)
- Electrostatics / Static Electricity
- Engines (5)
- Force and Motion
- Heat (72)
- Light / Optics
- Liquids and Air Pressure (33)
- Magnetism (41)
- Measurement (17)
- Nuclear Physics
-
Rocketry Rockets
- Air Rockets (2)
- Estes Rockets
- Helicopters R/C (1)
- Water Rockets (1)
- Sound & Waves (22)
- Spectrology (39)
- Technology (2)
- Timers (16)
- Weights and Hangers (47)
- Plasticware
- Porcelain Ware
- Safety Equipment
- Software / Multimedia
- STEM Kits
- Timers (16)
- UV Ultraviolet (1)
Powered by X-Cart shopping-cart-software
Copyright © 2005-2026 Science Lab Supplies